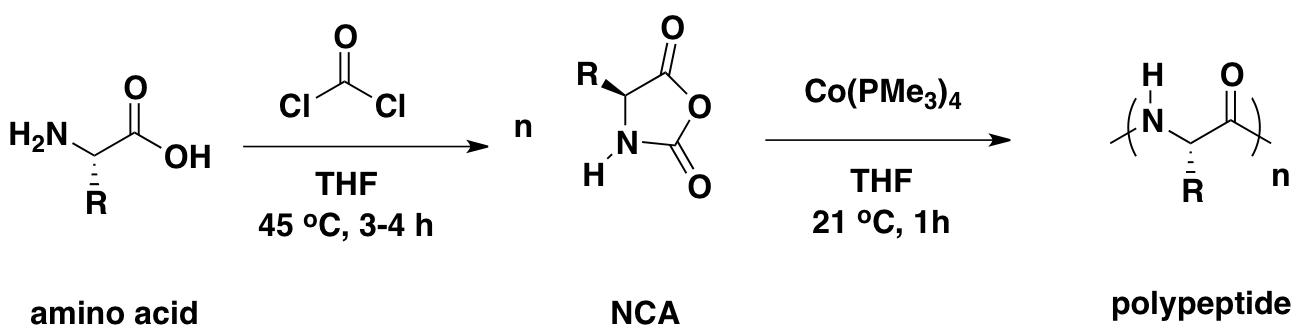
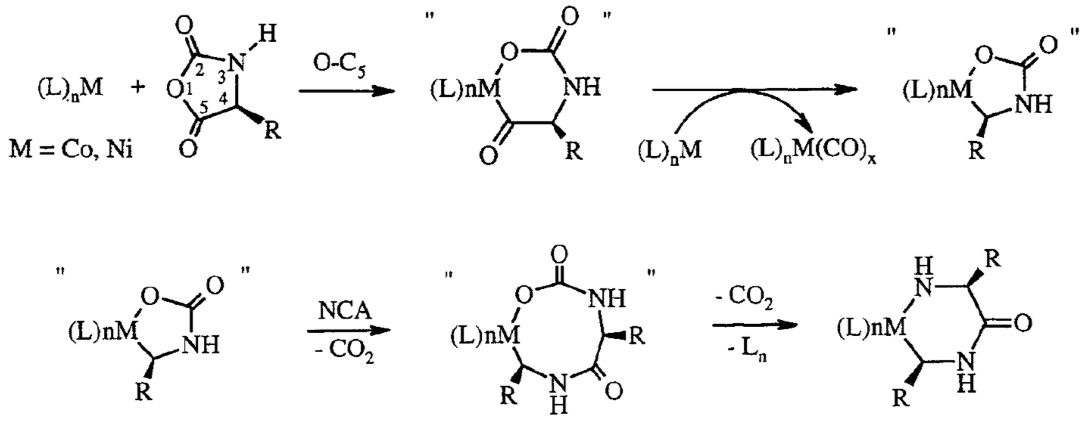
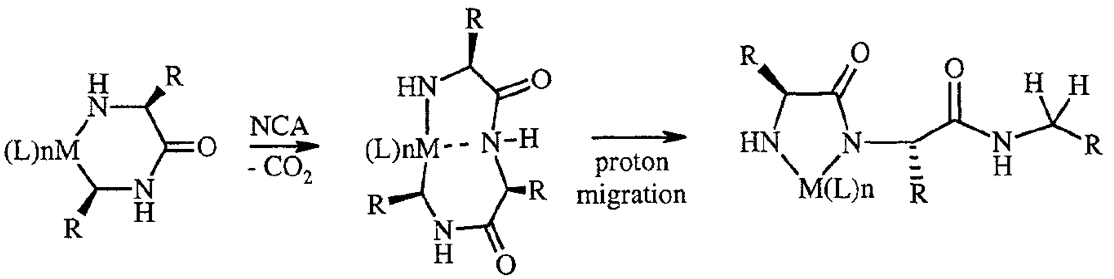
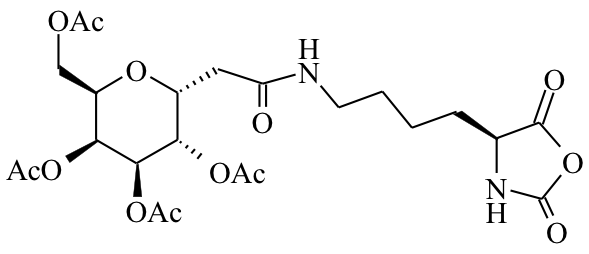
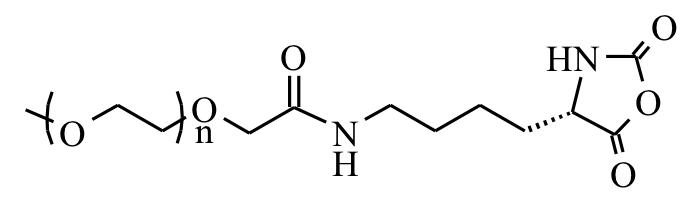
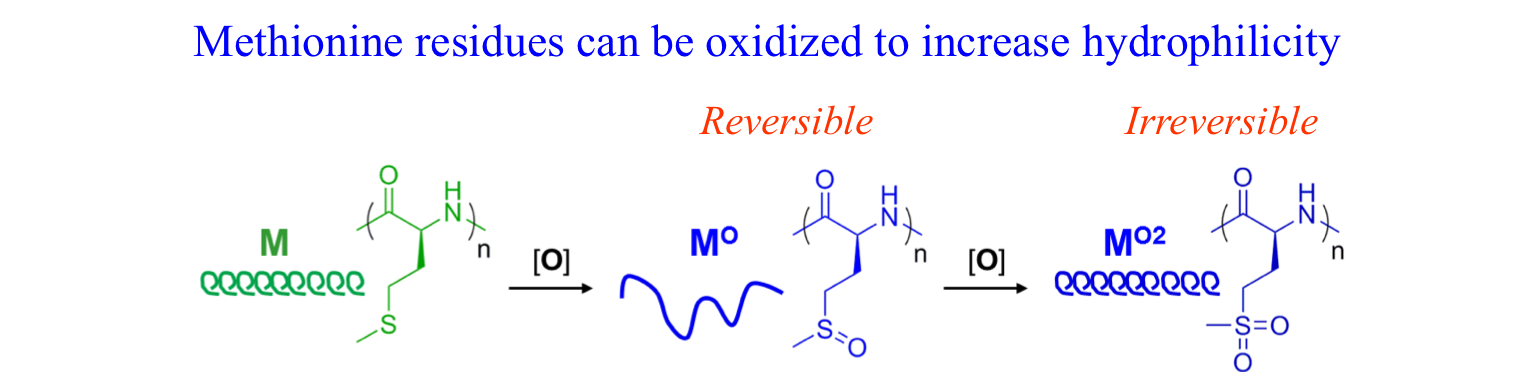
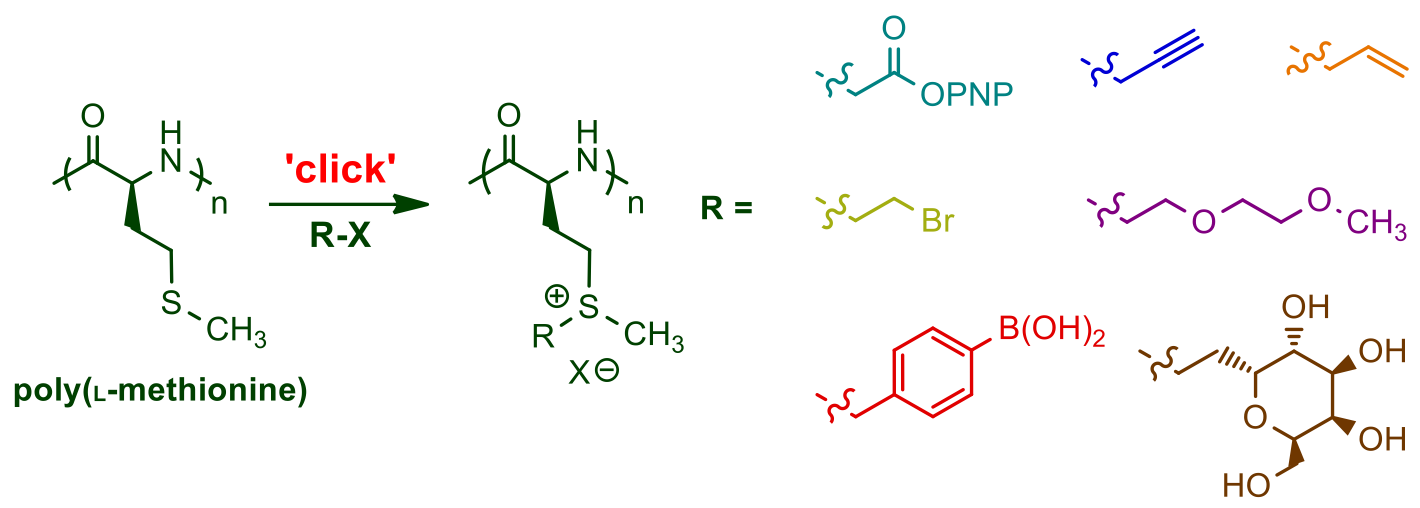
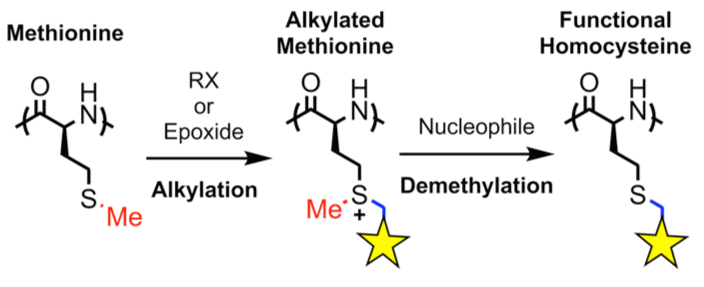
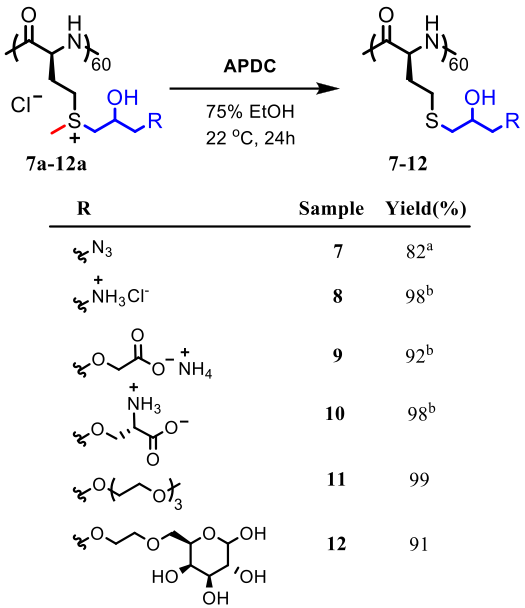
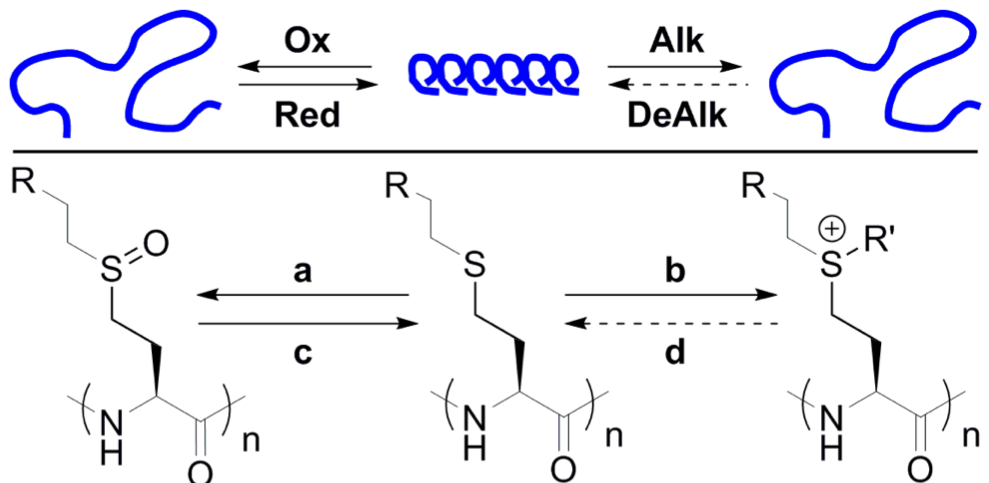
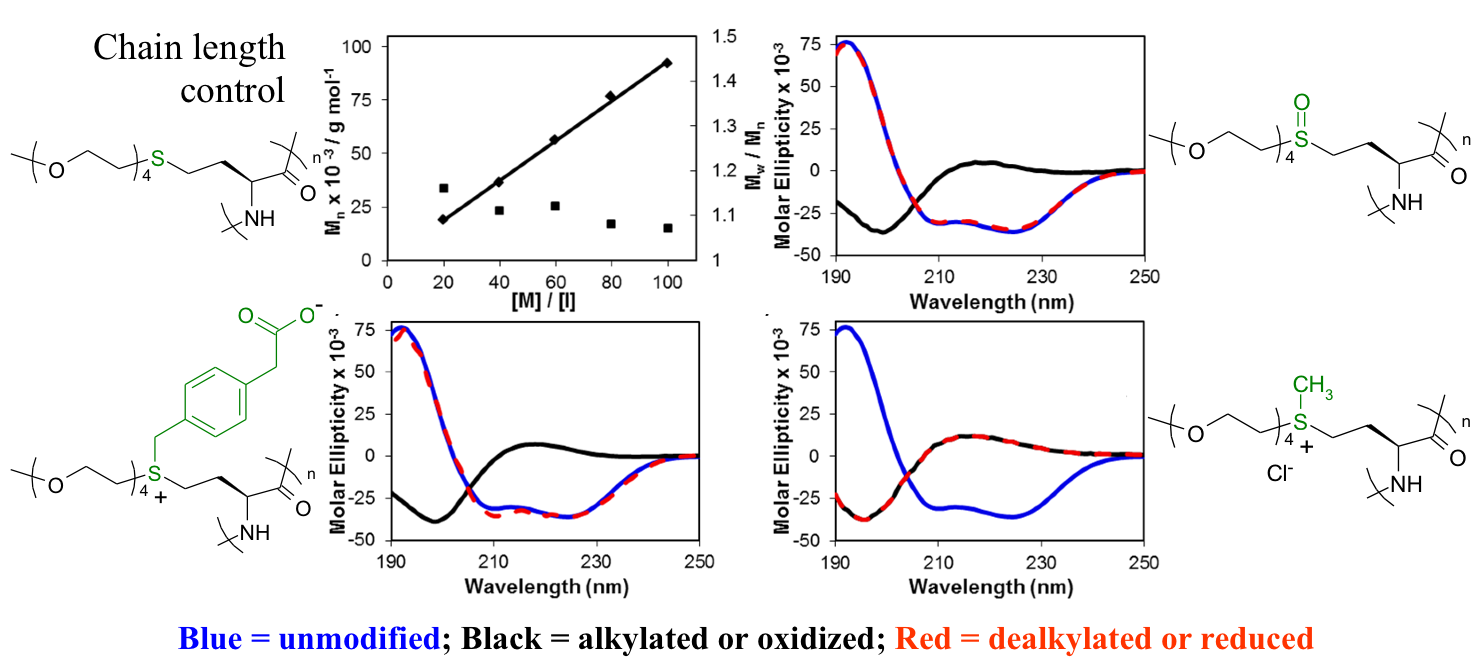
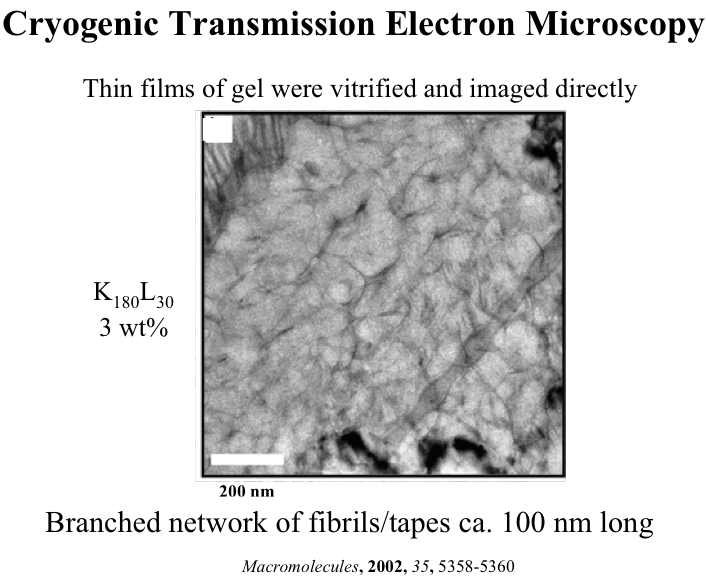
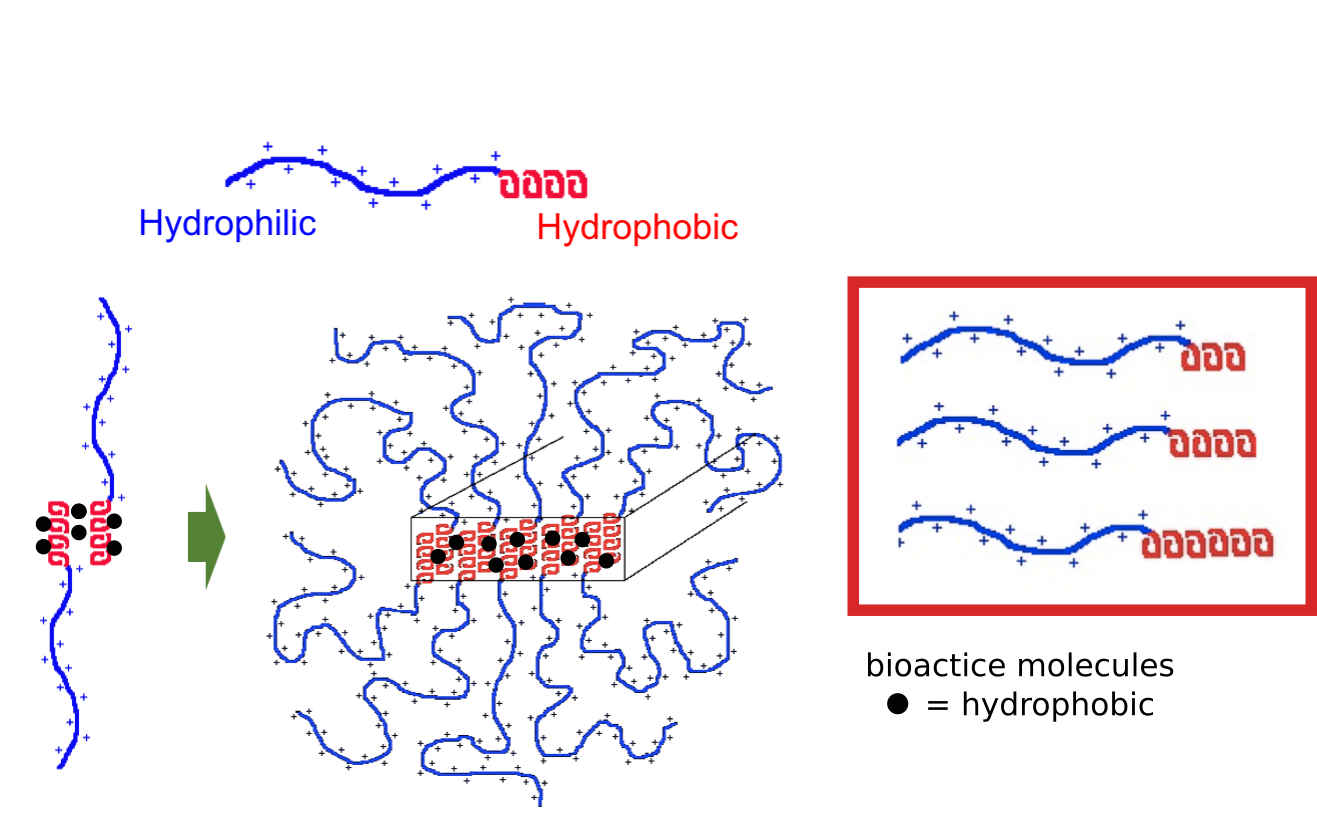
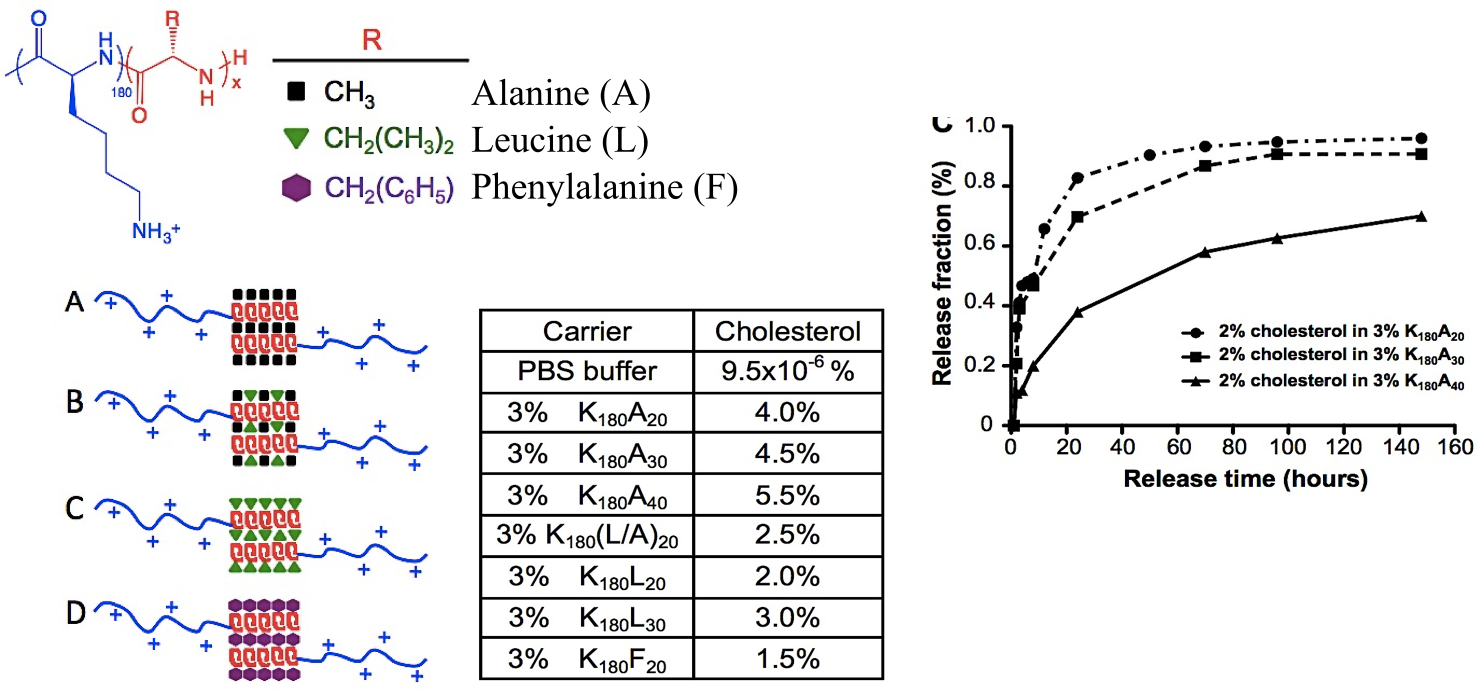
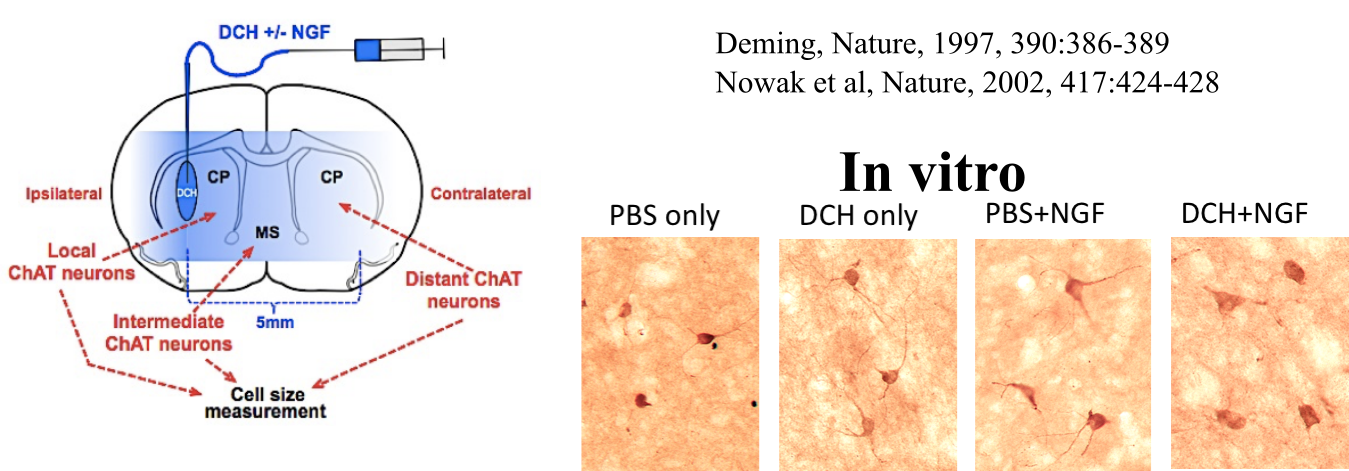
Polyion Complex DCH (DCHPIC)
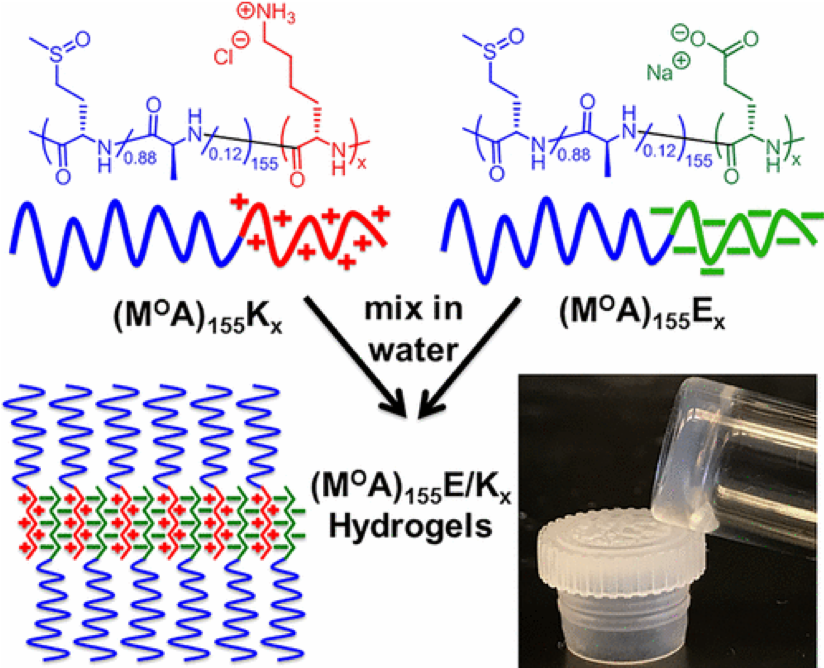
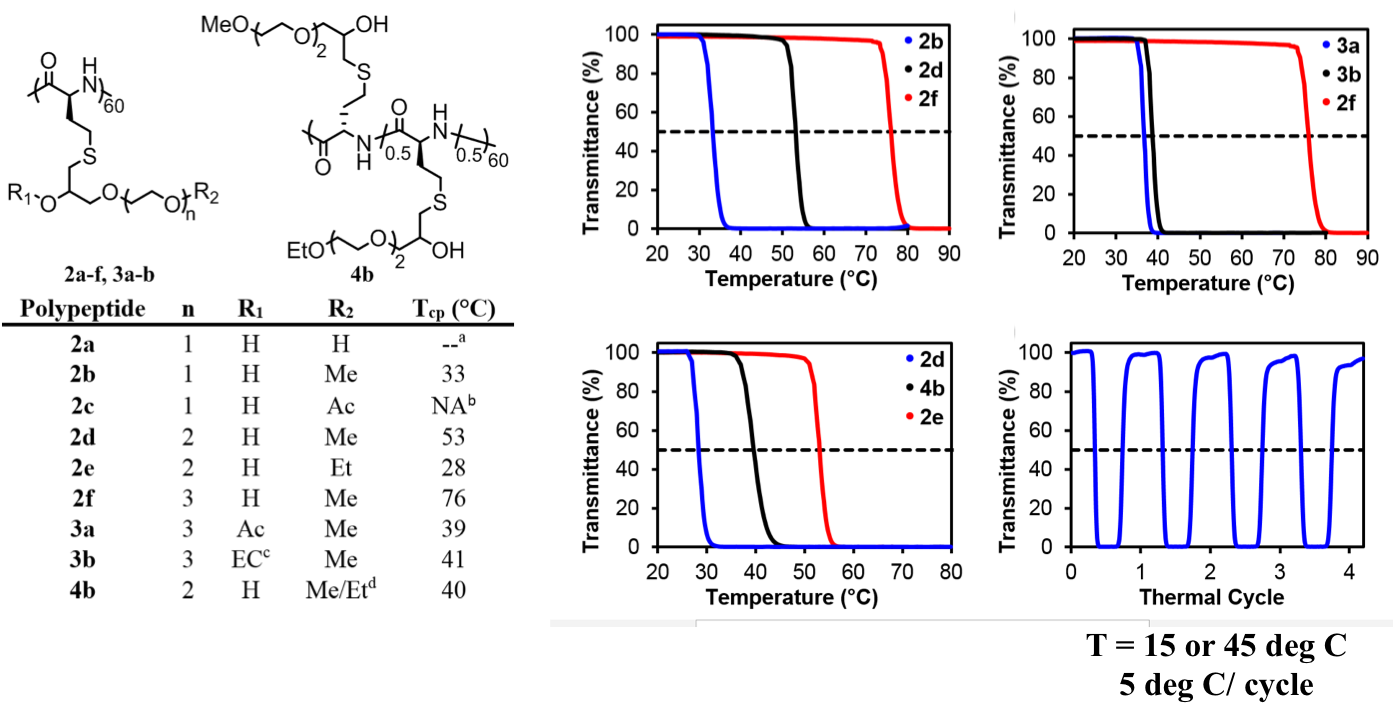
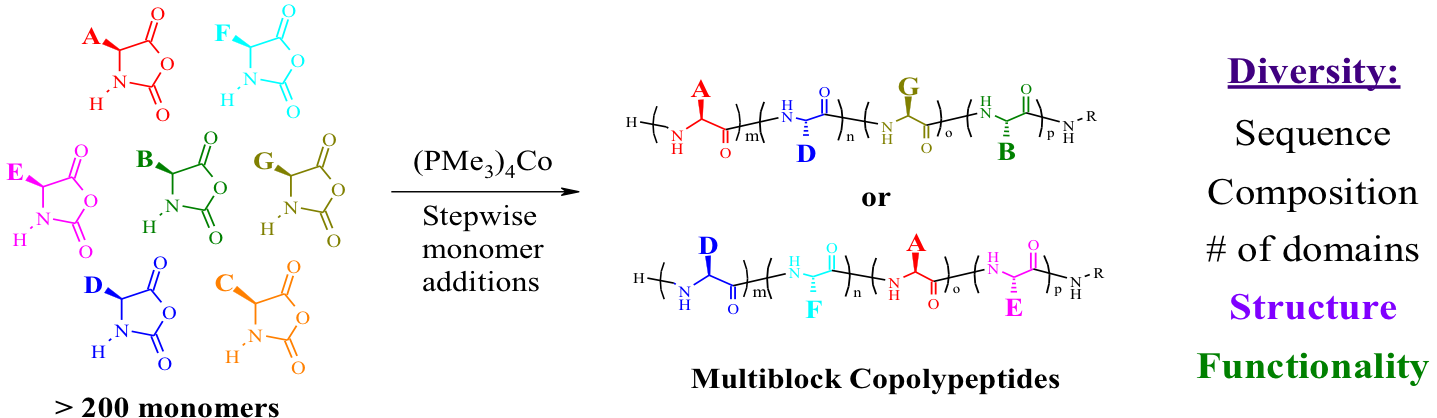
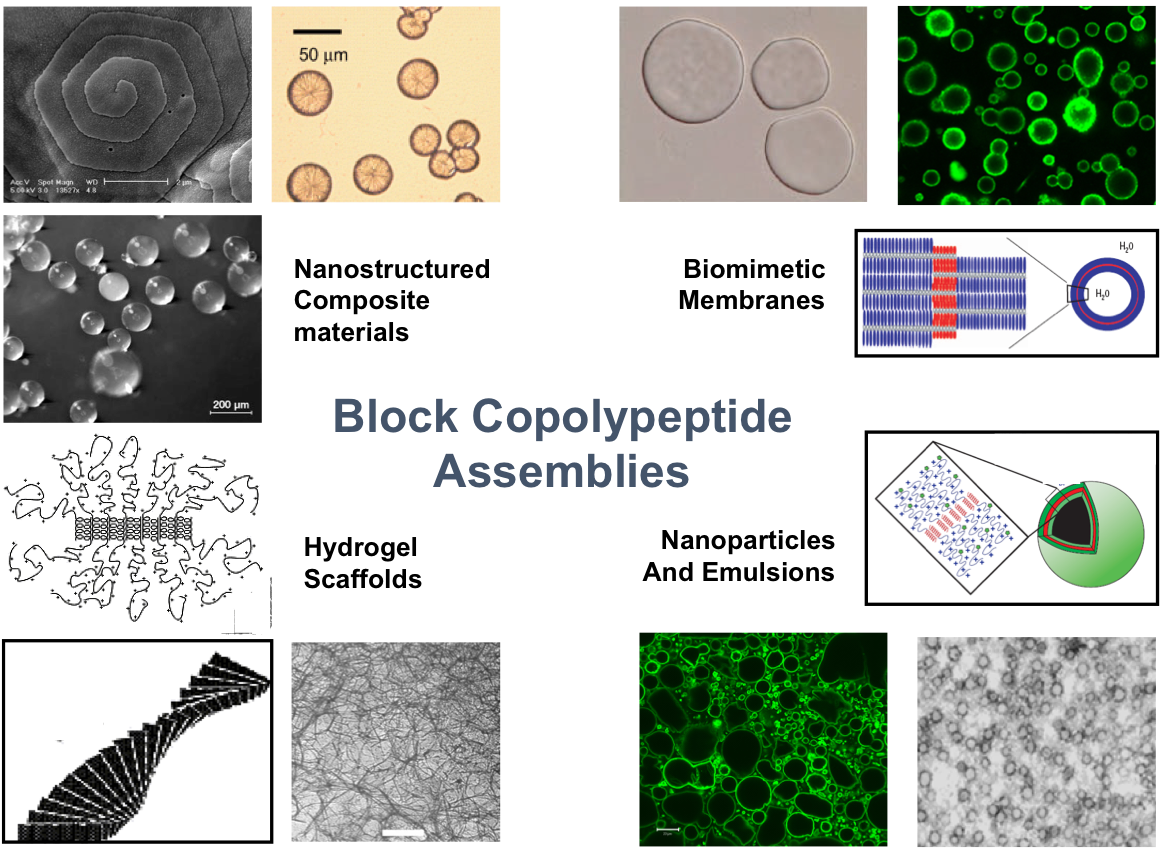
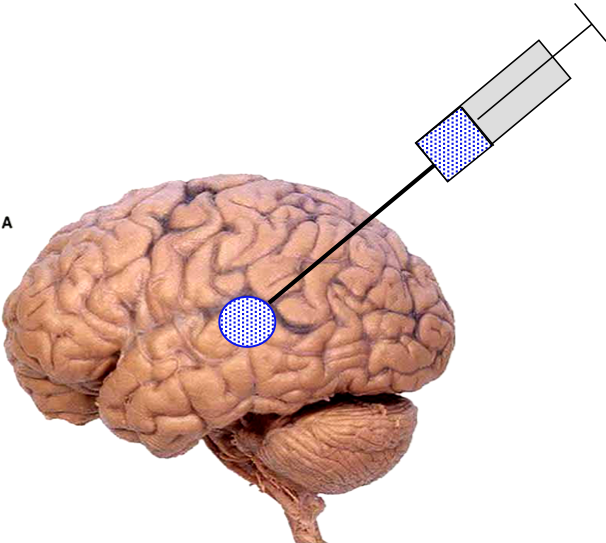
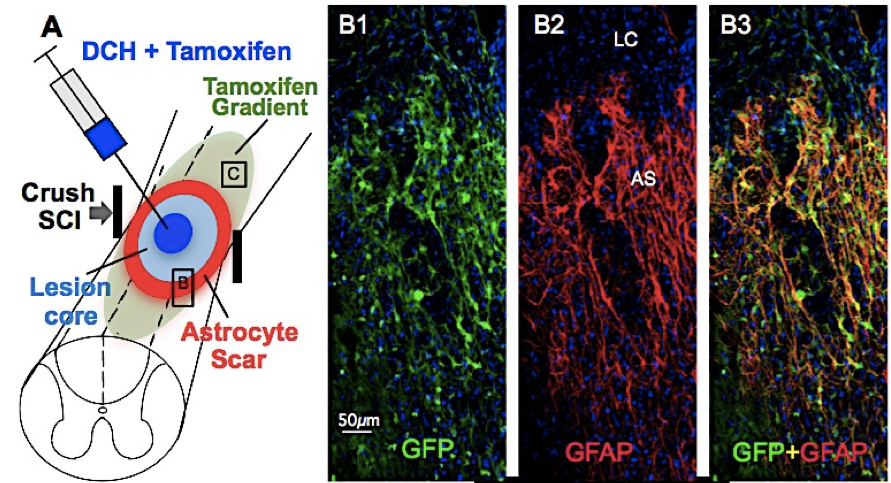
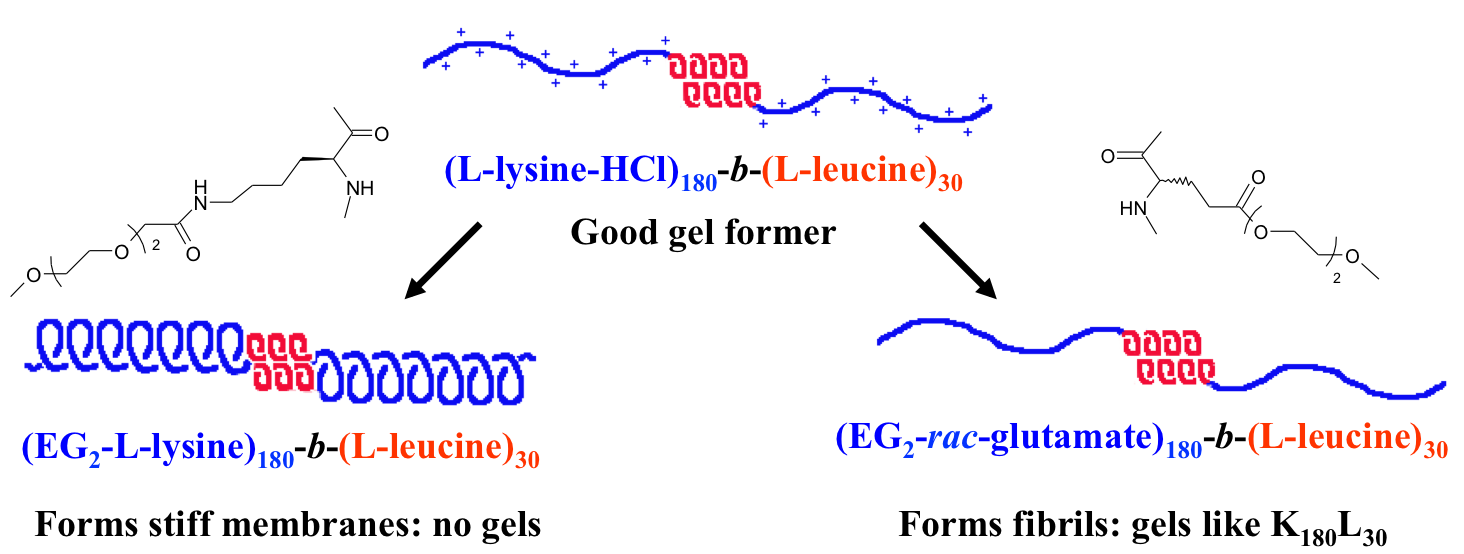
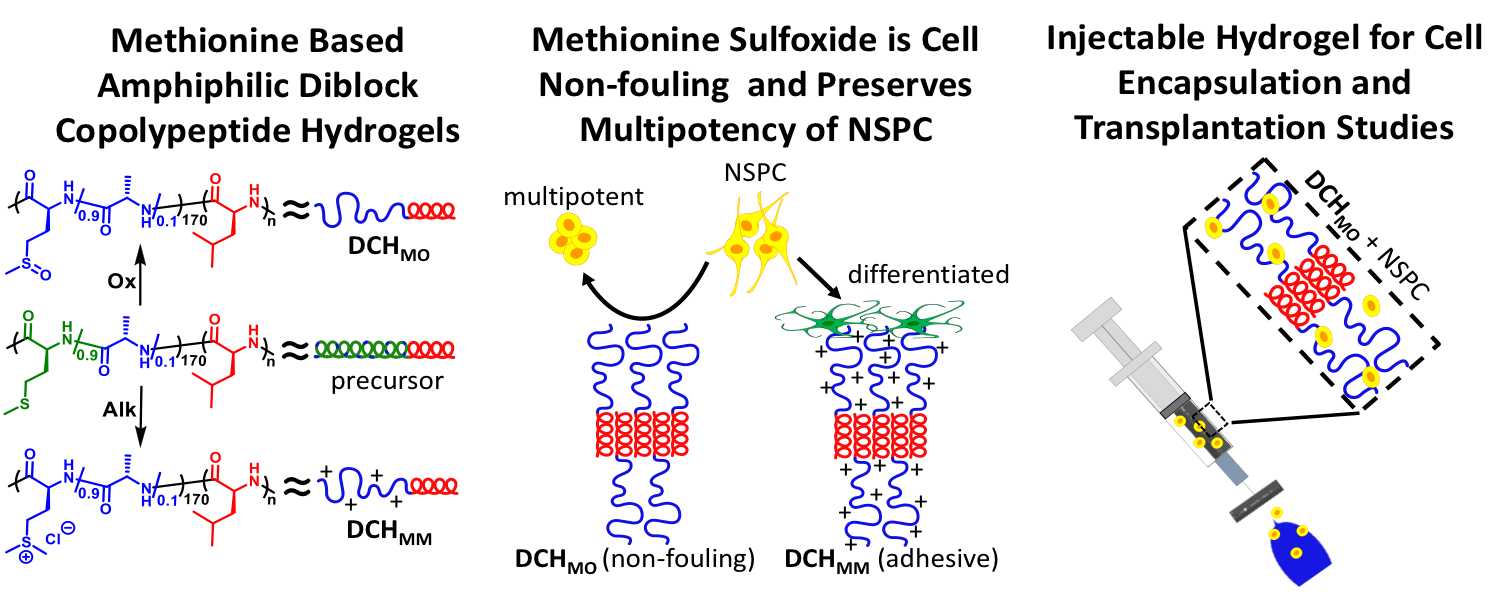
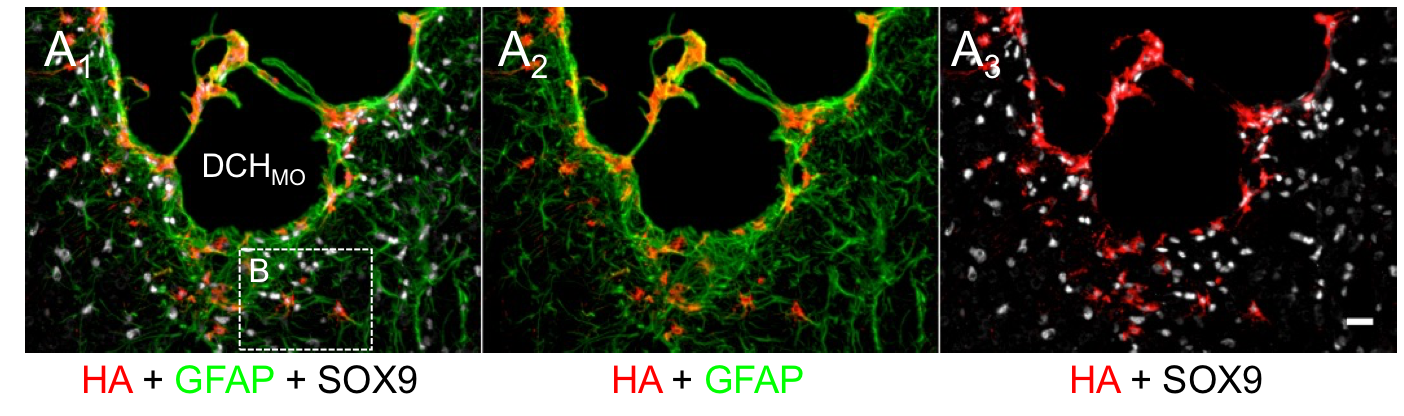
Another recent development has been the design of DCH that can assemble via polyion complexation as opposed to hydrophobic interactions. By taking advantage of the ability of enantiomerically pure poly(lysine) and poly(glutamate) segments to form beta-sheet structured polyion complexes, we were able to form DCH that retain the functionality of DCHMO, but are assembled via stronger electrostatic and H-bonding interactions. The result is DCHPIC that are significantly more resistant to dissolution compared to DCHMO, and can be prepared with much greater stiffness as two-component formulations while retaining cell and tissue compatibility.
Sun, Y.; Wollenberg, A. L.; O'Shea, T. M.; Cui, Y.; Zhou, Z. H.; Sofroniew, M. V.; Deming, T. J. J. Amer. Chem. Soc., 2017, 139, 15114-15121.