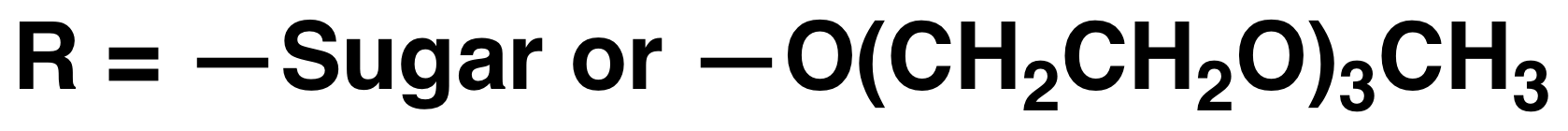Introducing Biological Functionality into Polypeptides
Synthesis of Functionalized NCAs
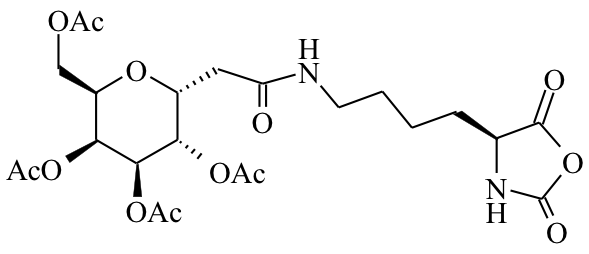
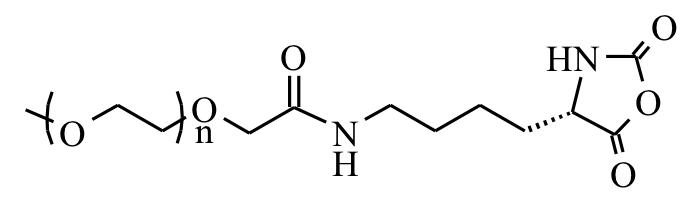
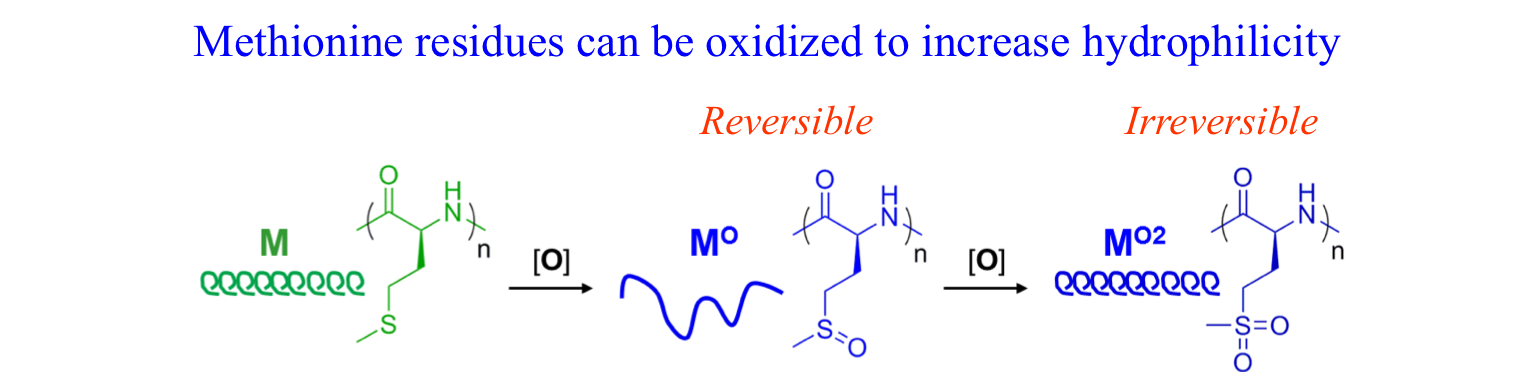
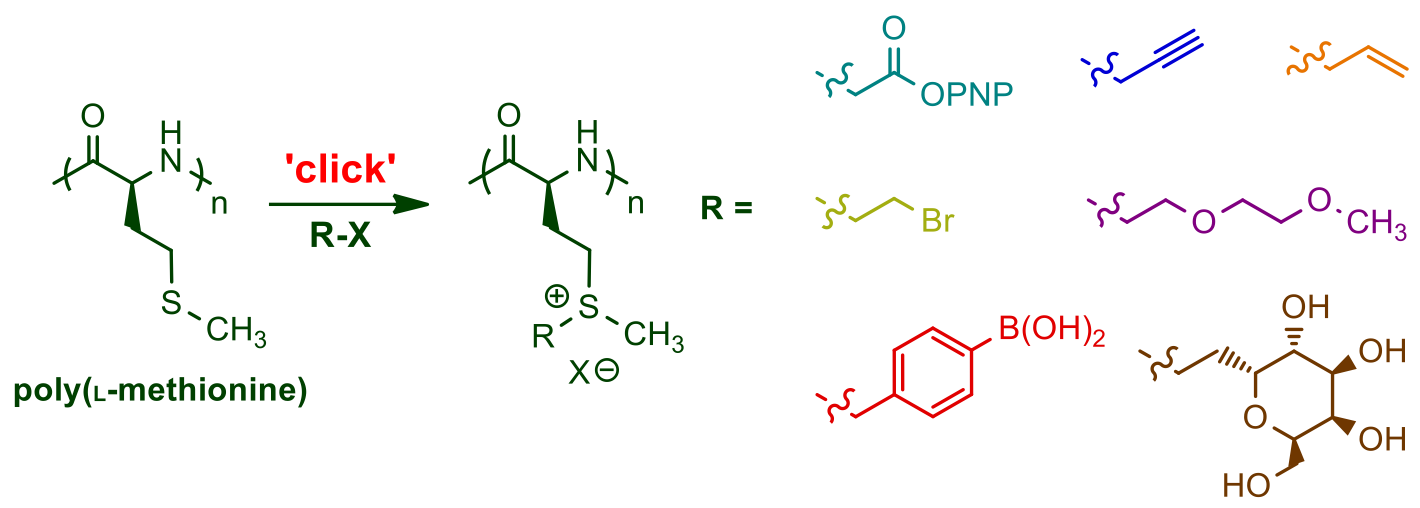
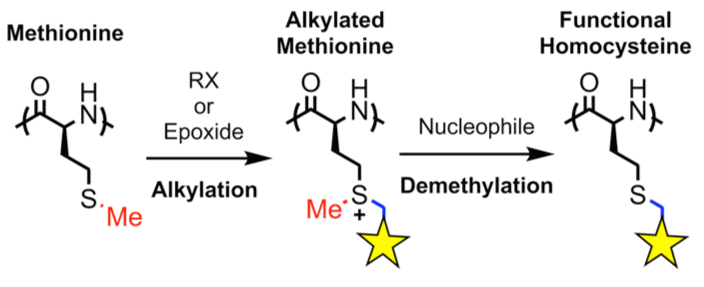
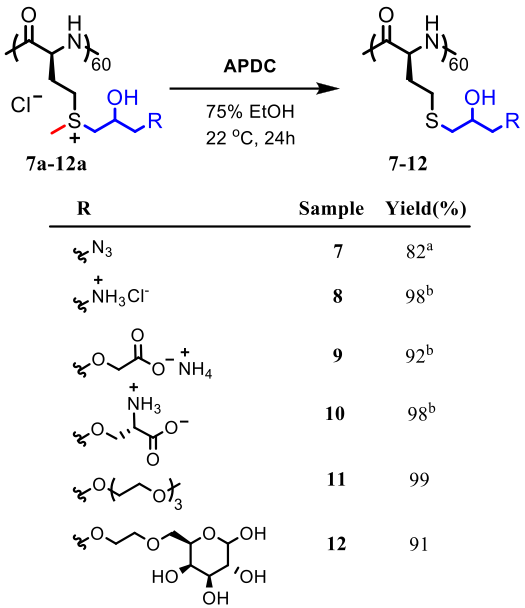
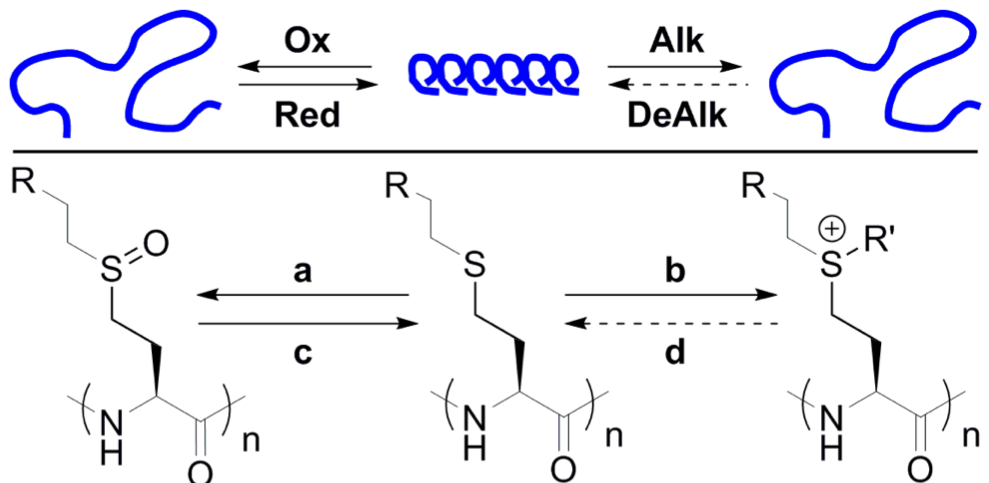
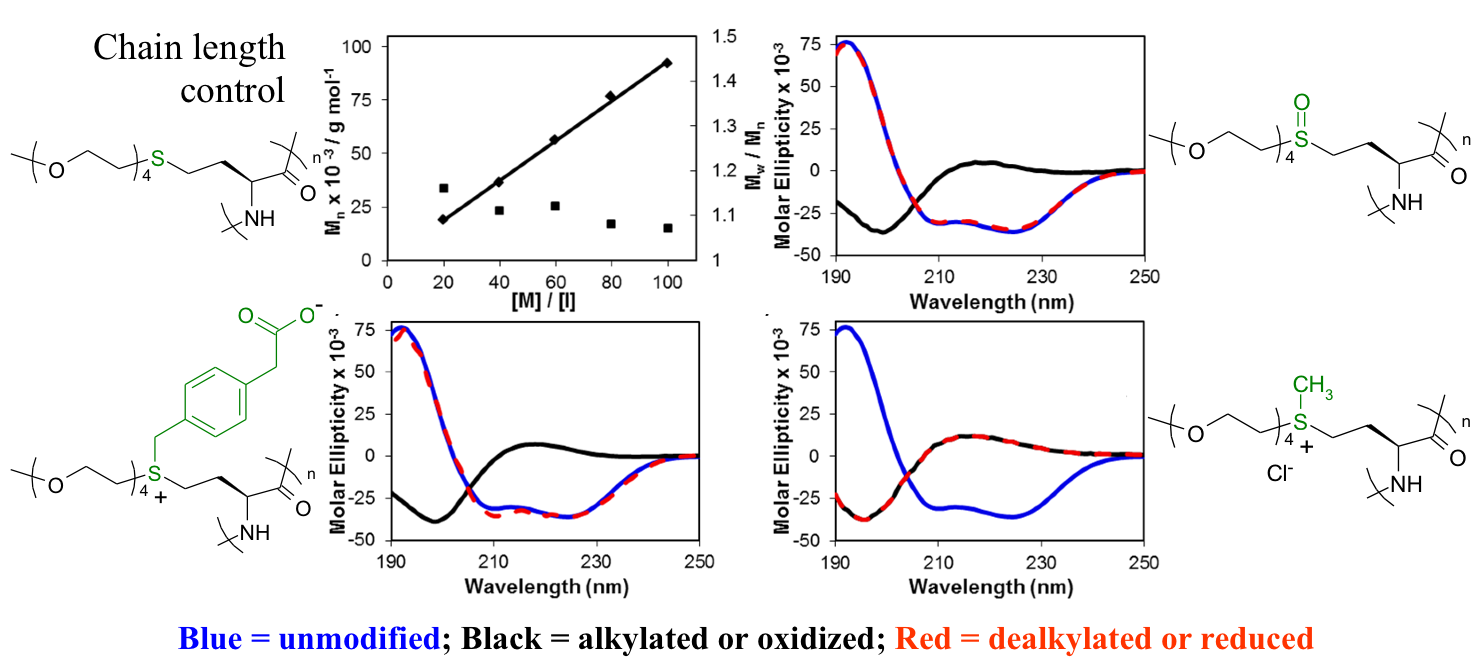
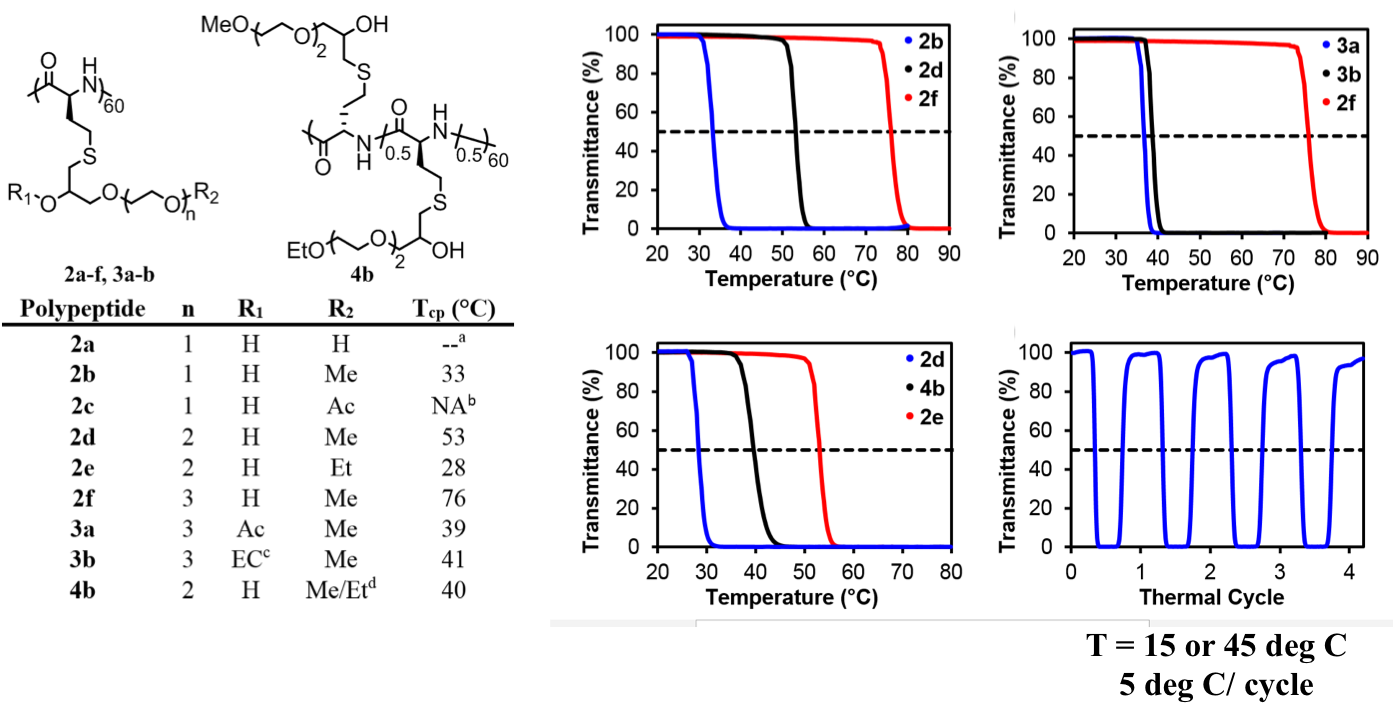
The use of functionalized NCAs allows direct incorporation of biologically relevant functional groups in precise locations in chains, but may require multiple synthetic steps and tedious purification.
Perlin, P.; Gharakhanian, E. G.; Deming, T. J. Chem. Commun., 2018, 54, 6196 - 6199.
Deming, T. J. Chem. Rev. 2016, 116, 786-808.
Yakovlev, I.; Deming, T. J. J. Amer. Chem. Soc., 2015, 137, 4078-4081.
Yakovlev, I.; Deming, T. J. ACS Macro Lett., 2014, 3, 378-381.
Rhodes, A. J.; Deming, T. J. ACS Macro Lett., 2013, 2, 351-354.
Rhodes, A. J.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 19463-19467.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2010, 132, 15068-15071.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2010, 11, 3668-3672.
Deming, T. J. Chem. Rev. 2016, 116, 786-808.
Yakovlev, I.; Deming, T. J. J. Amer. Chem. Soc., 2015, 137, 4078-4081.
Yakovlev, I.; Deming, T. J. ACS Macro Lett., 2014, 3, 378-381.
Rhodes, A. J.; Deming, T. J. ACS Macro Lett., 2013, 2, 351-354.
Rhodes, A. J.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 19463-19467.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2012, 134, 4112-4115.
Kramer, J. R.; Deming, T. J. J. Amer. Chem. Soc., 2010, 132, 15068-15071.
Kramer, J. R.; Deming, T. J. Biomacromolecules, 2010, 11, 3668-3672.