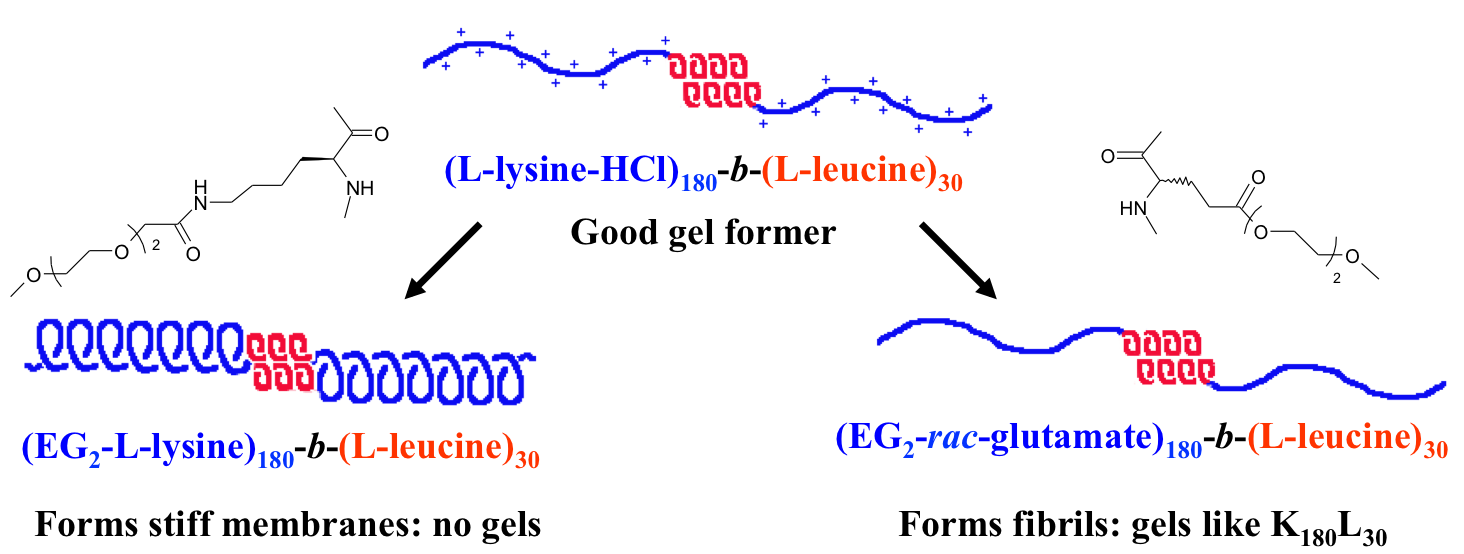
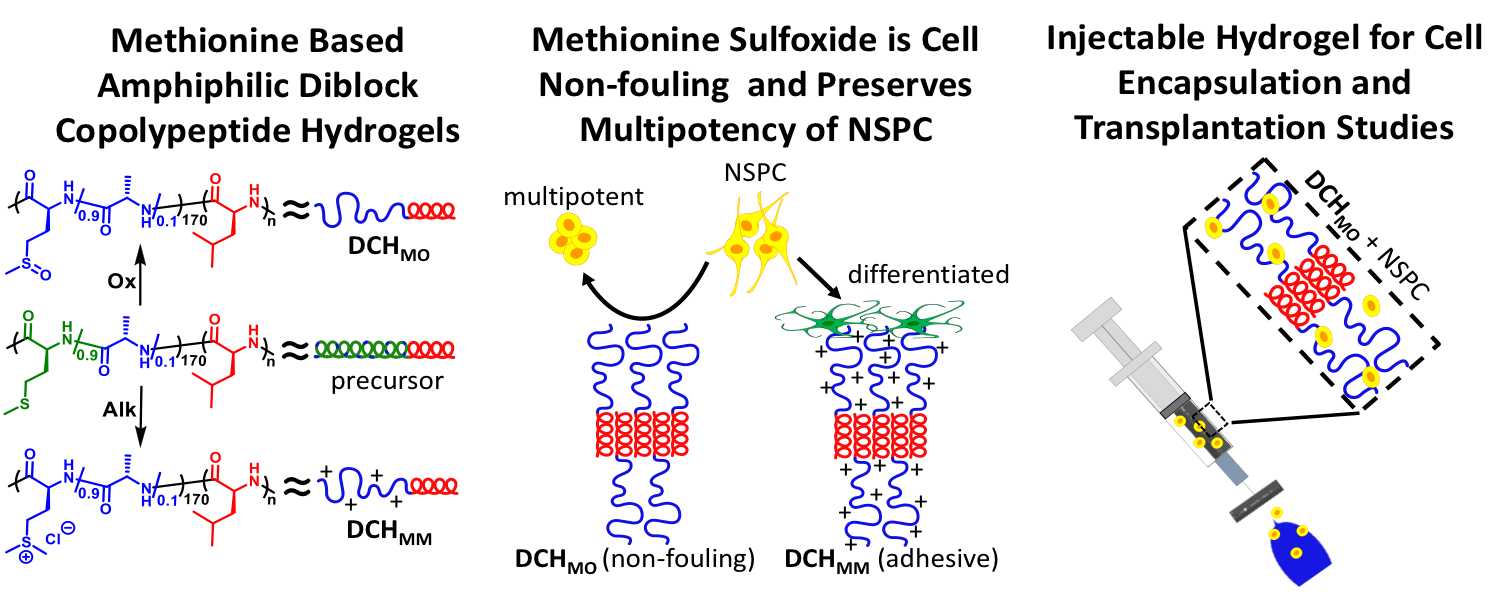
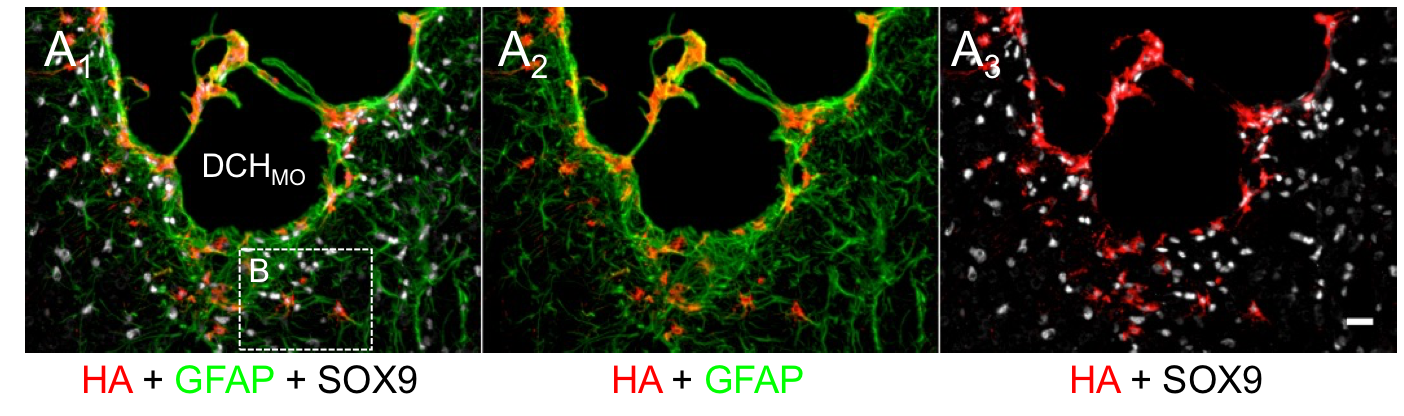
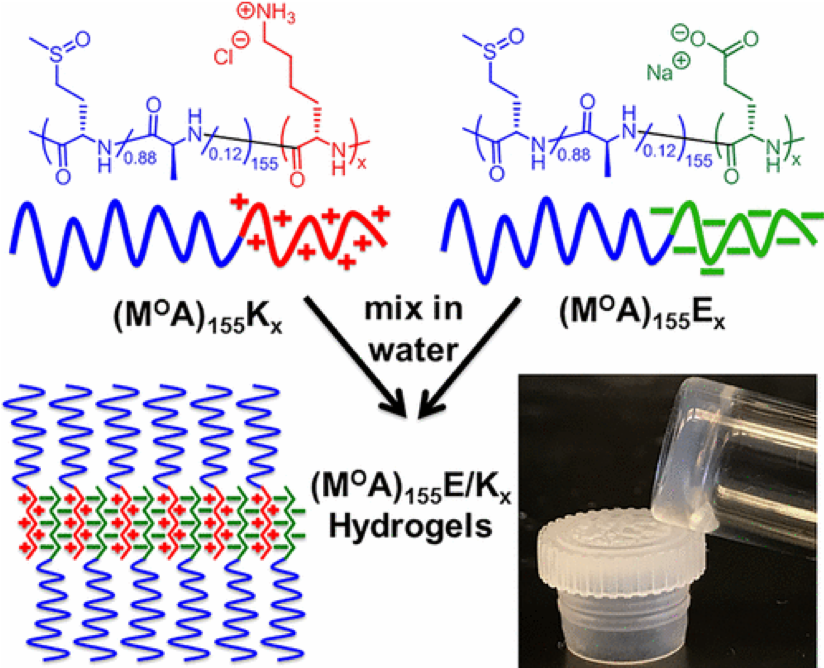
Diblock Copolypeptide Hydrogels (DCH) for Central Nervous System Repair
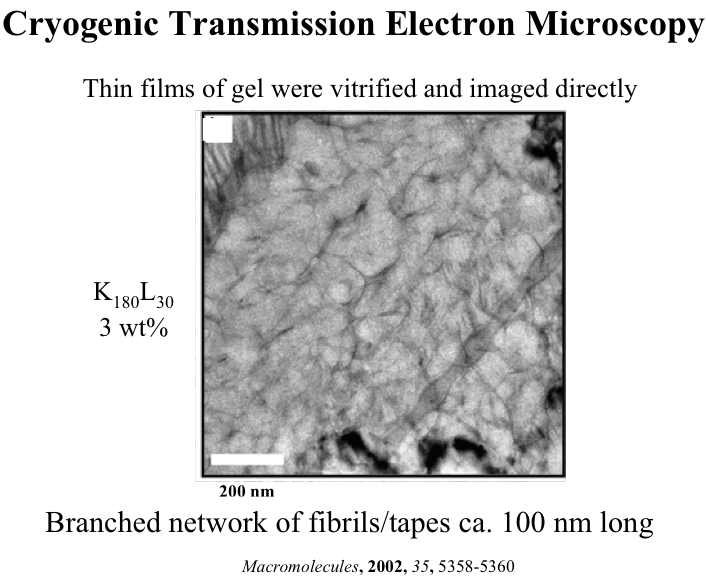
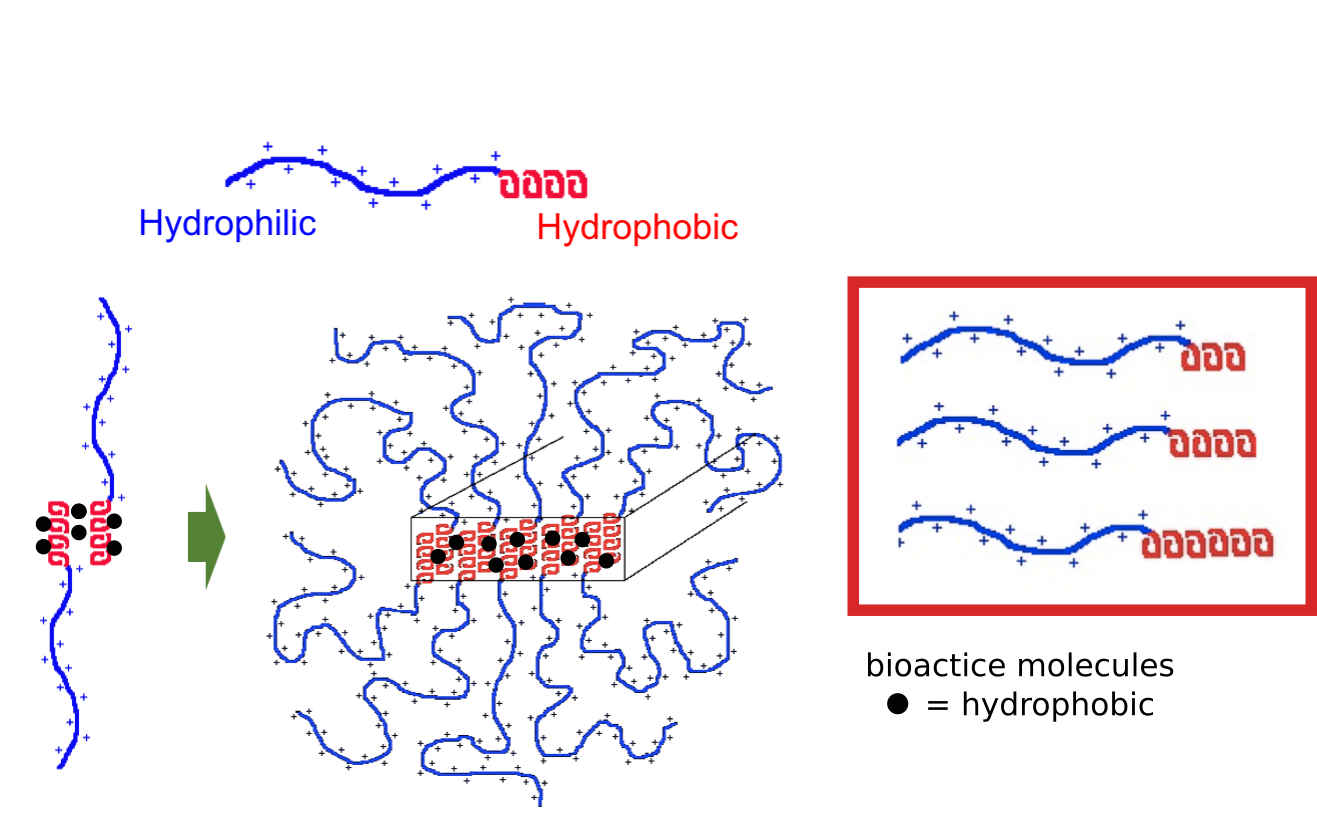
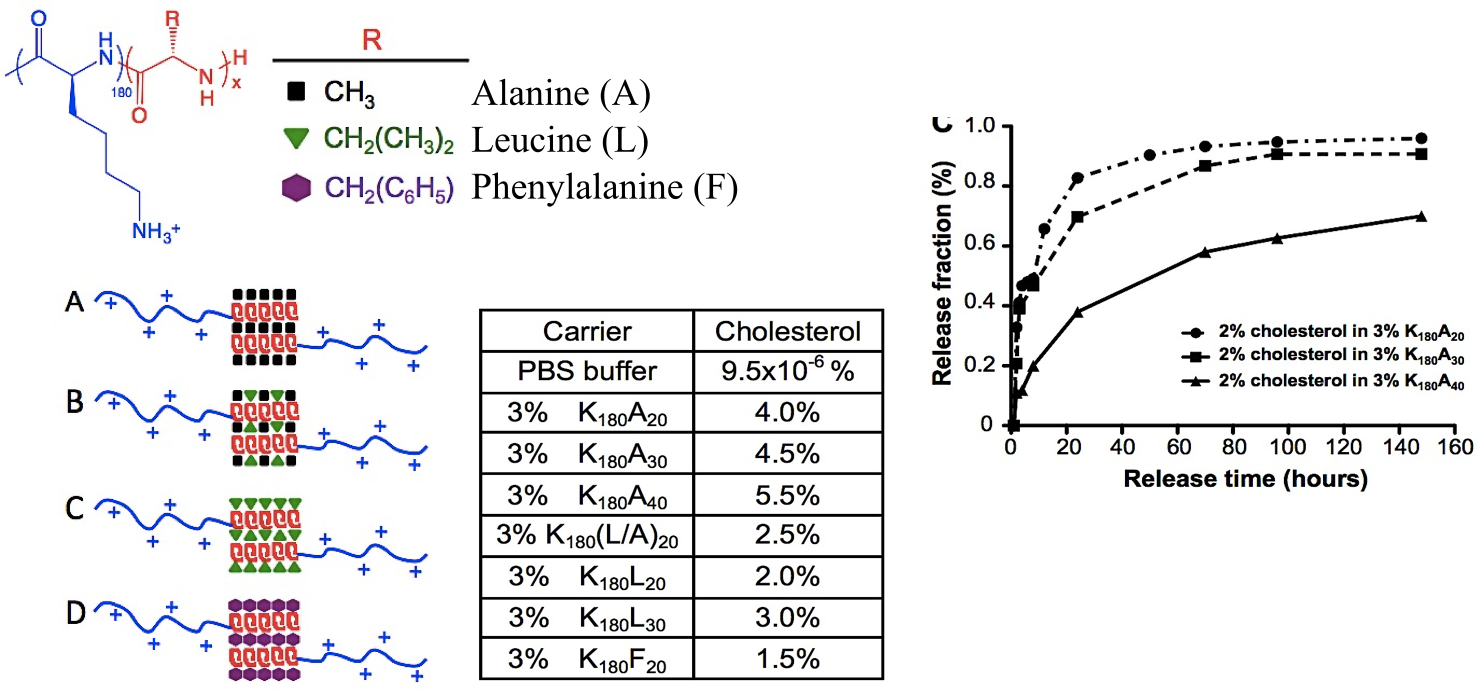
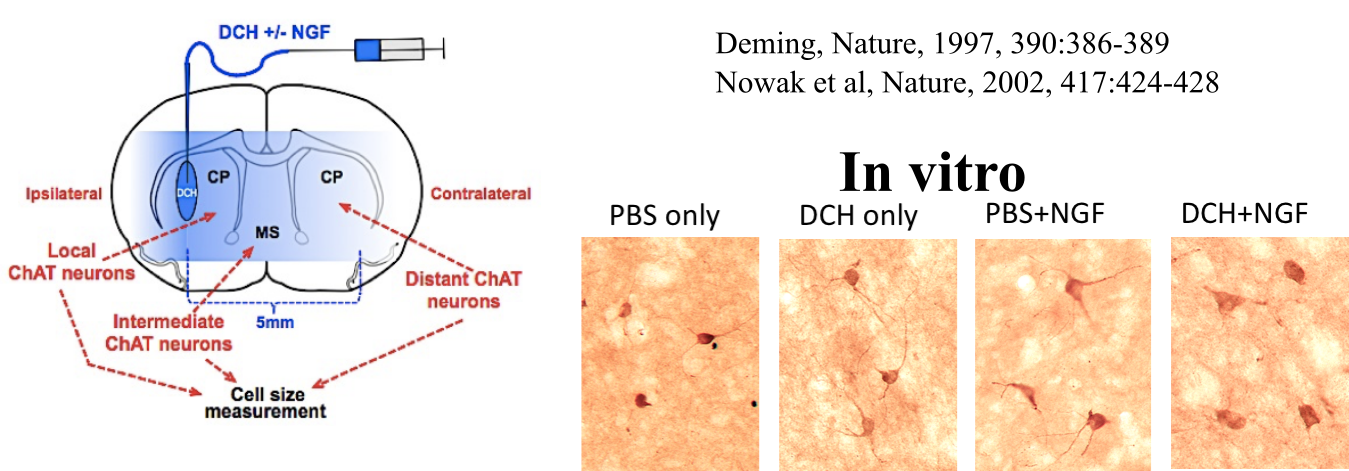
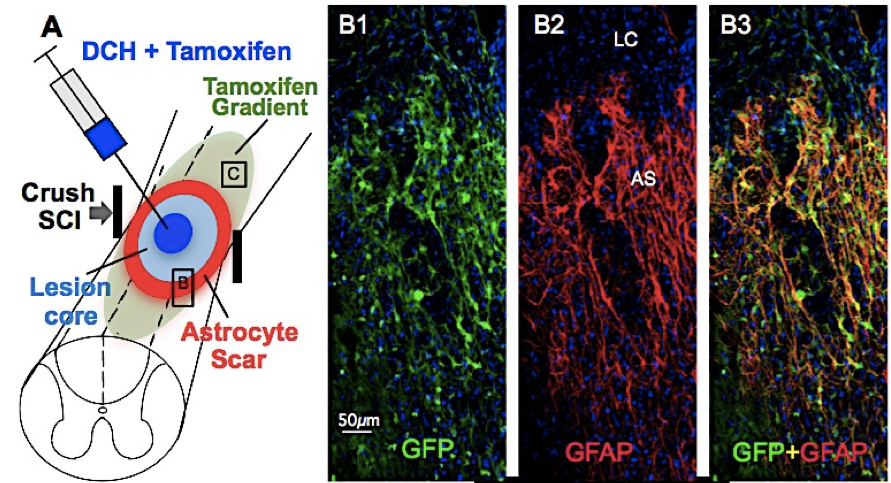
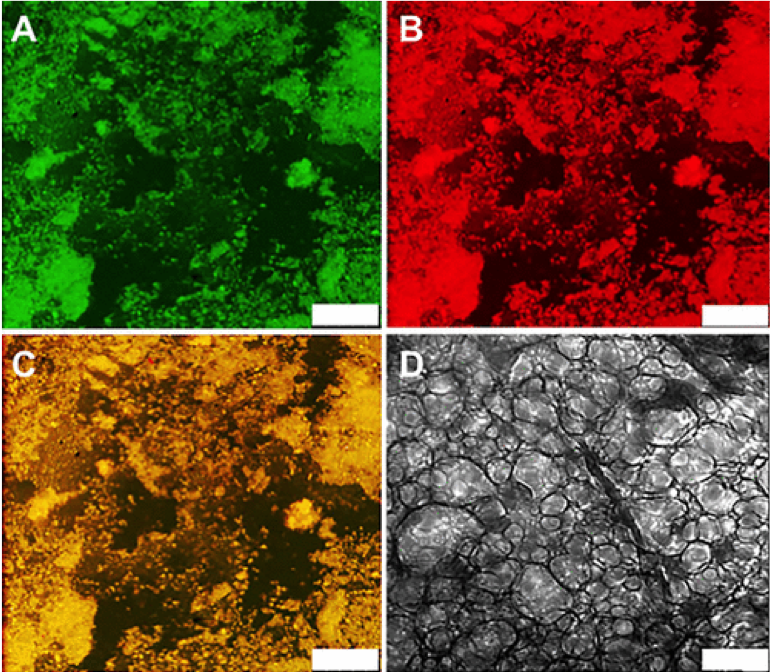
In collaboration with the lab of Prof. Michael Sofroniew (Neurobiology Dept, UCLA), were are developing polypeptide hydrogels for study of biology and neural repair in central nervous system (CNS) tissues. Synthetic DCH have been designed with tunable physical properties (stiffness, porosity, chemical functionality), and can be degraded in vivo. We have shown that many DCH formulations form hydrogel deposits and are well tolerated in healthy mouse forebrain tissue. DCH are being developed to encapsulate and release both hydrophilic (e.g. protein) and hydrophobic (e.g. small molecule active agent) cargos within CNS tissues. We are also developing DCH formulations to encapsulate neural progenitor stem cells (NPSCs) to allow cell grafting within CNS tissues, and to provide biomimetic scaffolds for encapsulated cells. Recent efforts have focused on study and development of DCH formulations to facilitate neural repair after spinal cord injury.
Anderson, M. A.; O'Shea, T. M.; Burda, J. E.; Ao, Y.; Barlatey, S. L.; Bernstein, A. M.; Kim, J. H.; James, N. D.; Rogers, A.; Kato, B.; Wollenberg, A. L.; Kawaguchi, R.; Coppola, G.; Wang, C.; Deming, T. J.; He, Z.; Courtine, G.; Sofroniew, M. V. Nature, 2018, 561, 396-400.
Anderson, M. A.; Burda, J. E.; Ren, Y.; Ao, Y.; O'Shea, T. M.; Kawaguchi, R.; Coppola, G.; Khakh, B. S.; Deming, T. J.; Sofroniew, M. V. Nature, 2016, 532, 195-200.
Yang, C-Y.; Song, B.; Ao, Y.; Nowak, A. P.; Abelowitz, R. B.; Korsak, R. A.; Havton, L. A.; Deming, T. J.; Sofroniew, M. V. Biomaterials, 2009, 30, 2881-2898.